Definition Of Electrostatic Attraction
Definition Of Electrostatic Attraction. Electrostatic interaction (van der waals interaction ): Electrostatic attraction is the repulsion or attraction between two particles due to their charges and distance between these two charges.
Electrostatic interaction (van der waals interaction ): Like charges repel each other while unlike charges attract each other. Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing.
When Two Charged Objects Have Different Charges, They Will Exert Attractive Forces On Each Other That Try.
Coulomb force attraction or repulsion of particles or objects because of their electric charge. The amount of static charge that an object can hold depends on the physical properties of the material it is constructed with. The electrostatic bonding force is significant in chemistry because it bonds an ionic.
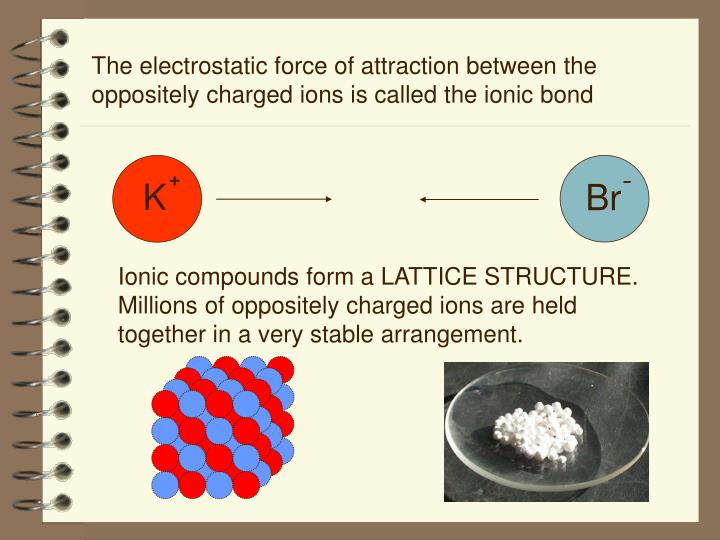
[Noun] A Chemical Bond Formed Between Oppositely Charged Species Because Of Their Mutual Electrostatic Attraction.
Electrostatic phenomena arise from the forces that electric charges exert on each other and are described by coulomb’s law. Force of attraction law and legal definition. Electrostatic attraction is the attraction between atoms of opposite charge that holds the atoms together in ionic bonds.
Intermolecular Forces Are Attractive Forces That Exist Between Different Molecules.
Electrostatics is a branch of physics that deals with the study of electromagnetic phenomena where electric charges are at rest, i.e., where no moving charges exist after a static equilibrium has been established. Even though electrostatically induced forces seem to be relatively weak. Coulomb's law is used to calculate the strength of the force between two charges.
Electrostatic Attraction Synonyms, Electrostatic Attraction Pronunciation, Electrostatic Attraction Translation, English Dictionary Definition Of Electrostatic Attraction.
Electrostatic repulsion occurs between two atoms of the same charge. The greek word for amber, ἤλεκτρον, was thus the source of the word 'electricity'. For example, the electrostatic force between protons and electrons in an atom is responsible for the atom’s stability.
The Strength Of The Electrostatic Forces Between Two Atoms Is Determined By The Size Of Each Atom’s Charge And The Distance Between The Two Atoms.
It’s also known as coulomb’s interaction or coulomb’s force. The second force that can cause attraction is the electric force also known as the electrostatic force. It's the attractive or repulsive force between two electrically charged objects.
Post a Comment for "Definition Of Electrostatic Attraction"